
The difference between the dipole moments in different solvents. The dipole moment in aliphatic amines is about 1 Debye and the direction is through the nitrogen directed to the lone pair. All the dipole moments calculated with B3LYP level were larger than that calculated with HF. (I have no idea, why the vector for the dipole got bigger and bigger, while the magnitude was decreasing.) The situation for any aliphatic amine can be expected to be very similar. If you have a close look at the HOMO, you will notice, that it only significantly extends to the first carbon.
I removed some of the charges to not overcrowd the image. In the series $\ce$, to example the trend. There is an online verison, but you can often find it in libraries, too.įor the meantime, I will try to illustrate something. If you are lucky enough to have access, the handbook of chemistry and physics has a large number of values. You can probably find tabulated values for most common compounds online. Of course you could go ahead and calculate the dipole moment from tabulated values of certain bonds ( for example), but this can be really tedious and it might now be always right. This difference in electronegativity generates a net dipole moment across the bond and makes it a polar. rotations of the alkyl chain and also the electron cloud. Higher electronegative atom attracts the shared bonded electrons slightly towards its side and gains a partial negative charge and other atom gains partial positive charge. Accurate TDDFT calculations of the dipole moment and components of the dipole polarizability for the ground and lowest excited states of uracil have been performed with a variety of functionals. It is very much dependent on how the molecule is currently shaped, i.e.

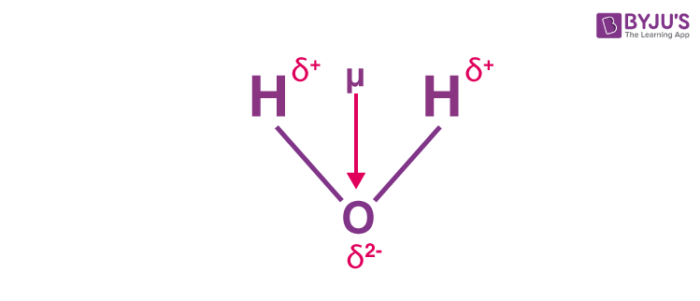
The total molecular dipole moment may be approximated as the vector sum of the individual bond dipole moments.
HF DIPOLE MOMENT VERIFICATION
page needed verification needed For polyatomic molecules, there is more than one bond. I really do not see an easy and straight forward way of determining the dipole moment's direction and quantity. 2, has zero dipole moment, while near the other extreme, gas phase potassium bromide, KBr, which is highly ionic, has a dipole moment of 10.41 D.


 0 kommentar(er)
0 kommentar(er)
